Carbon emissions from the industrial sector accounts for 30% of U.S. emissions. These emissions can be greatly reduced through the use of “green” hydrogen, i.e., hydrogen produced via the electrolysis of water with renewable energy.
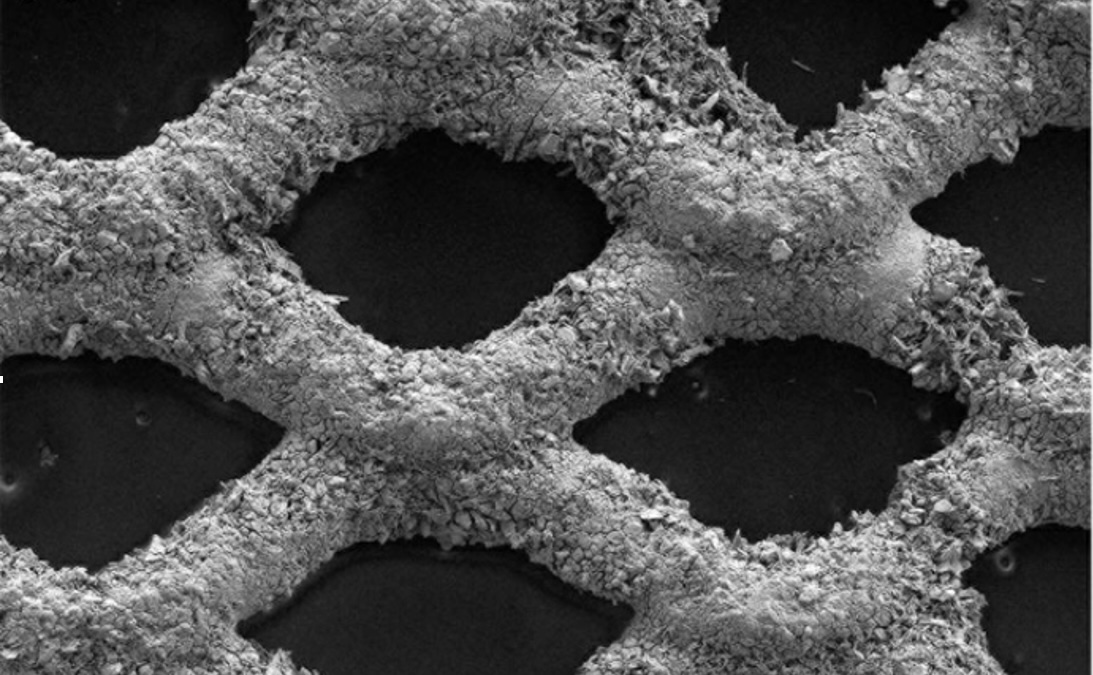
The problem with green hydrogen is its high cost, about $4.2 per kg, vs. $2 per kg for “gray” hydrogen (from steam methane reforming). The biggest contributors to the cost of green hydrogen are the cost of electricity and the cost of the electrolyzer. Research in the Wiley lab has focused on how to decrease these costs by increasing the electrolyzer’s productivity (kg/hr of H2). Most recently, the Wiley lab described how to achieve a 50-fold increase in the maximum rate of hydrogen production over conventional alkaline electrolyzers through the use of a microfibrous flow-through electrode.
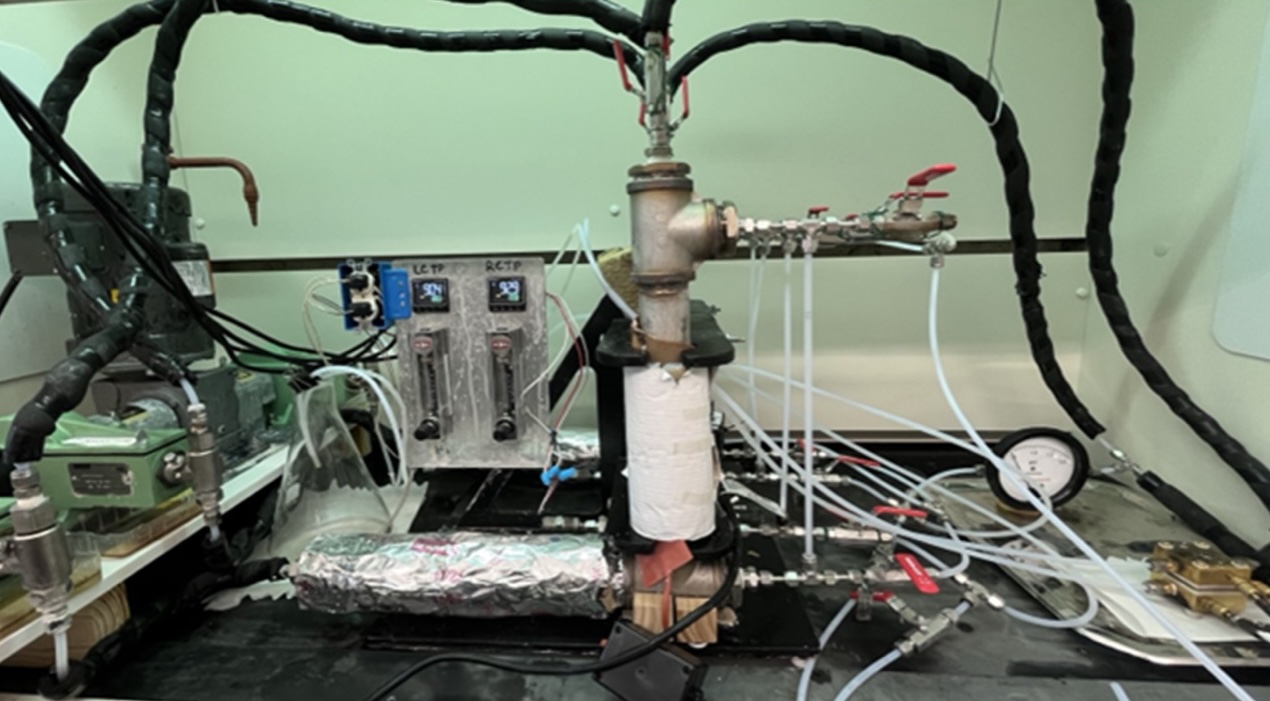
We are building upon our previous efforts to improve the efficiency, productivity and durability of electrodes for alkaline electrolysis. To date we have achieved 2 A cm-2 at 2 V with 32wt.% KOH, 90°C, 150 psi, and with a Zirfon UTP PERL 220 membrane. Durability tests are ongoing.
Related Publications:
