When syntheses of metal nanocrystals with different shapes (cube, rod, prism) were first reported early in the 21st century, researchers noted that it was surprising that such anisotropic nanostructures could be grown from metals with symmetric cubic crystal structures. One of the most frequently invoked hypotheses for why nanostructures grow anisotropically is the capping agent hypothesis, which proposes that the organic additives added to nanostructure syntheses are facet-selective in that they block atomic addition to certain facets but not others, leading to anisotropic growth. Despite many studies showing how different synthetic variables affect nanostructure morphology, as of 2016, there were not any conclusive tests of this facet-selective capping agent hypothesis.
We hypothesized that we could provide a definitive test of the capping agent hypothesis by performing single-crystal electrochemical experiments in the reaction solution. For the case of pentagonal nanowires, capping agents are thought to adsorb to the {100} facets on the sides of the nanowires, leaving the {111} facets on the ends open to atomic addition. If this hypothesis was correct, one would expect to see atomic addition to the surface of a (100)-oriented single crystal suppressed relative to the (111)-oriented surface of a single crystal.
We first applied the single-crystal electrochemistry methodology to the study of copper nanowires grown in 15 M NaOH with ethylenediamine (EDA) as a organic additive (JACS, 2017). The single-crystal electrochemistry experiments revealed that the capping agent hypothesis was false for this synthesis. Instead, EDA acted as a facet-selective promoter of copper nanowire growth by keeping the {111} facets on the ends of growing copper nanowires free of oxidation (see Figure 1). These results represent an important discovery in the field of nanostructure growth because they clearly showed (1) a facet-selective capping-agent hypothesis was false, and (2) organic additives could act as promoters (rather than passivators) of metal atom addition to a nanostructure. However, it is unlikely that the results of this study on the EDA-mediated synthesis of Cu nanowires can be generalized to other syntheses of metal nanostructures as most syntheses do not take place in highly basic solutions. In addition, most metal nanostructure syntheses require the use of halides, which were absent in this particular copper nanowire synthesis.
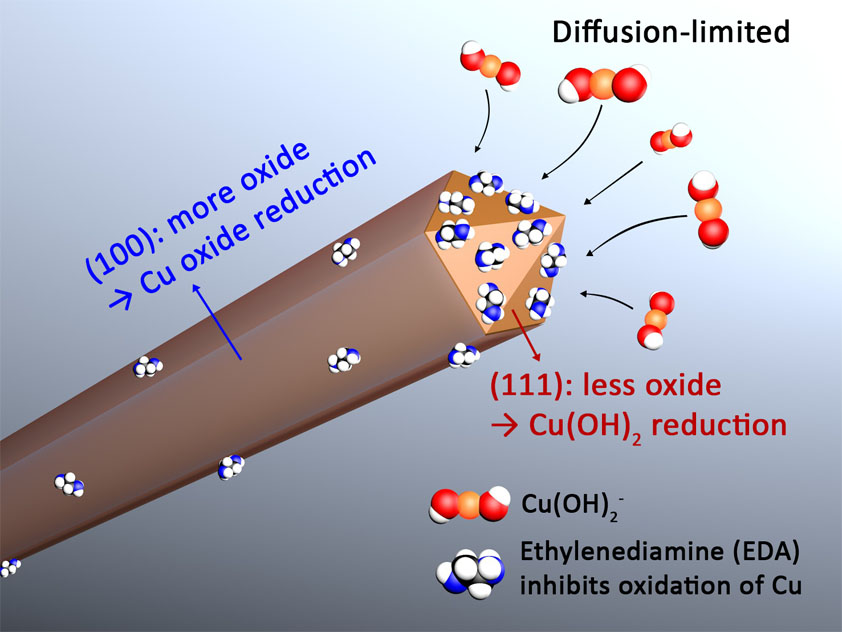
Figure 1. EDA acts as a facet-selective promoter of copper nanowire growth by keeping the {111} facets on the ends of growing copper nanowires free of oxidation. See the paper for more details
To address this potential lack of generality for the EDA-mediated mechanism and probe the role of halides, we subsequently applied the single-crystal methodology to a synthesis that involved the growth of copper nanowires in the presence of chloride at neutral pH (JACS, 2018). 17 This study focused on answering the question of why chloride is necessary for the anisotropic growth of Cu nanowires when hexadecylamine (HDA) is used as a capping agent (see Figure 2A-C). Single-crystal electrochemistry experiments revealed that HDA by itself does not act as a capping agent. Only the combination of HDA with chloride enables selective deposition of copper onto the {111} facets on the ends of copper nanowires. Chloride disrupts the ability of the HDA monolayer to passivate Cu(111) at lower concentrations than for Cu(100) (Figure 2D). The concentration of chloride that caused the greatest difference in charge transfer resistance between the Cu(111) and Cu(100) exactly matched the concentration that produced copper nanowires with the highest aspect ratio. The strong correlation between the single-crystal electrochemistry and synthetic results indicated that similar facet-selective chemistry was likely occurring in both cases.
Further insight into why chloride displaced HDA from Cu(111) was provided by density functional theory (DFT) calculations from our collaborator Kristen Fichthorn at Penn State (Figure 2E). Both Cu(100) and Cu(111) exhibited similar N-Cu bond distances (and thus similar bond strengths) in the absence of chloride, which explains the formation of spherical particles in the absence of chloride (Figure 2B). At a chloride coverage of 0.25 monolayers, the Cu-N interaction doubled in strength for Cu(100), but weakened for Cu(111). Increasing the chloride coverage to 0.33 monolayer resulted in weak physisorption of HDA on Cu(111) due to short-range repulsion between chloride and HDA, whereas the structure of Cu(100) accommodated strong chemisorption of both chloride and HDA. Higher concentrations of chloride displaced HDA from both facets. Prof. Fichthorn’s DFT calculations confirmed that HDA bonding is facet-dependent in a narrow range of chloride concentrations.
The corroborating evidence from Cu nanowire synthesis, single-crystal electrochemistry, and DFT calculations provided strong support for the proposed growth mechanism illustrated in Figure 2F. An intermediate concentration of chloride led to selective desorption of HDA from the {111} facets at the ends of the Cu nanowires, thereby causing anisotropic growth. This work indicates the simple explanation of HDA acting as a facet-selective capping agent was incomplete. Instead, chloride selectively displaced HDA from {111} facets. This work further illustrates that the combination of nanostructure synthesis, single-crystal electrochemistry, and DFT can lead to an atomic level understanding of how to control the addition of atoms to specific facets.
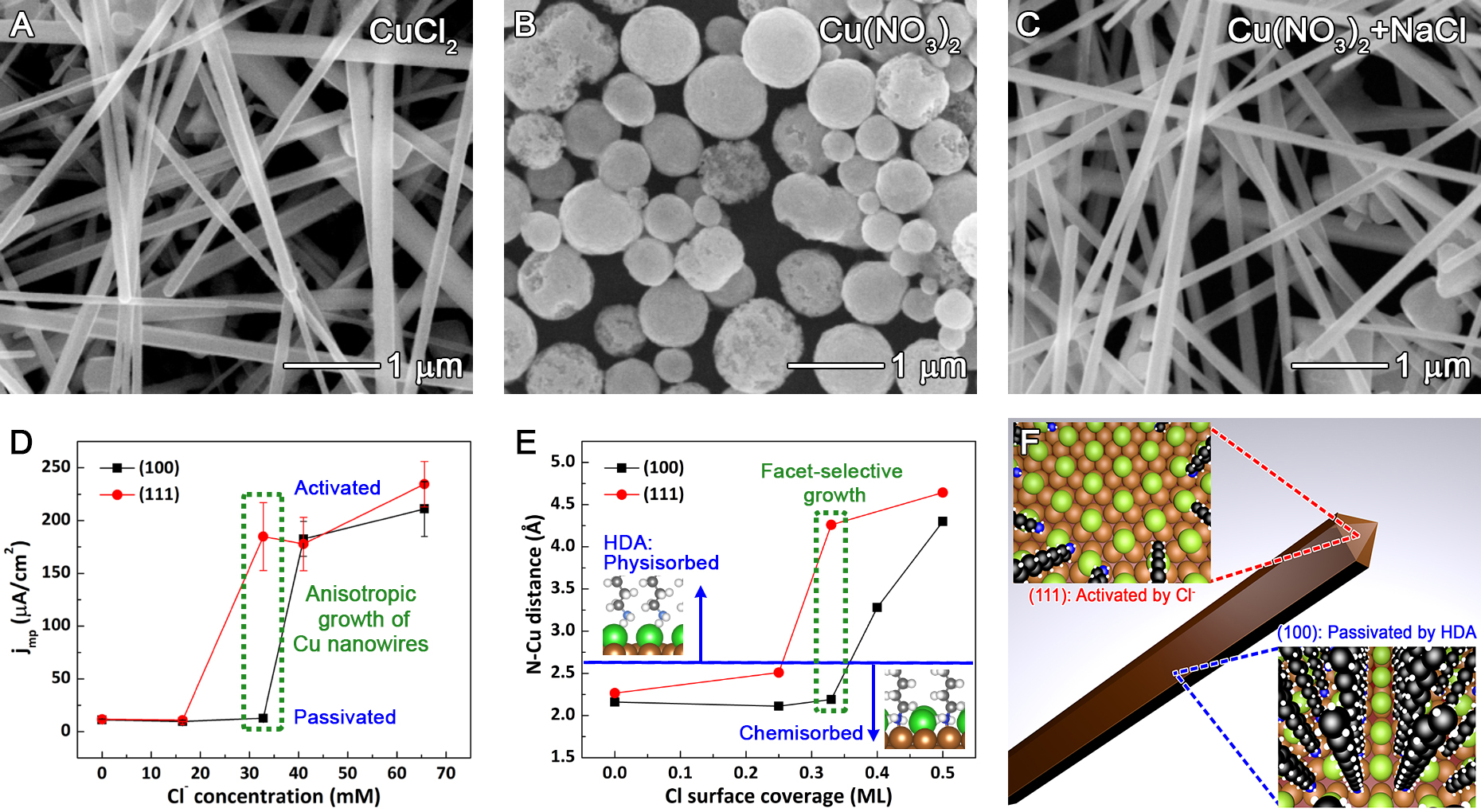
Figure 2. (A-C) Synthetic results with and without chloride in the presence of HDA (HDA is present in each case). Cu nanowires only grow in the presence of chloride and HDA, and do not grow without chloride. (D) Current at the mixed potential, where the oxidation rate of the reducing agent matches the rate of Cu(II) reduction, as a function of chloride concentration for Cu(111) and Cu(100) single-crystal electrodes. (D) DFT results for competitive adsorption of HDA and chloride on Cu(100) and Cu(111). Chlorine atoms are shown in green. (F) A schematic diagram showing the proposed growth mechanism of Cu nanowires in the presence of HDA and chloride. See the paper for more details
Related Publications:
Kim, M.J.; Flowers, P.F.; Stewart, I.E.; Ye, S.; Baek, S.; Kim, J.J., Wiley, B.J.; Ethylenediamine Promotes Cu Nanowire Growth by Inhibiting Oxidation of Cu(111). J. Am. Chem. Soc., 2017, 139, 277.
Kim, M.J.; Alvarez, S.; Chen, Z.; Fichthorn, K.A.; Wiley, B.J. Single-Crystal Electrochemistry Reveals Why Metal Nanowires Grow. J. Am. Chem. Soc., 2018, 140, 14740.
Huo, D.; Kim, M.J.; Lyu, Z.; Shi, Y.; Wiley, B.J.; Xia, Y. One-Dimensional Metal Nanostructures: From Colloidal Syntheses to Applications. Chem. Rev., 2019, ASAP.