For many metals, it is currently not possible to synthesize high yields of nanowires in a wide range of dimensions. We sought to circumvent this problem by coating copper nanowires with different metals. Copper nanowires have the advantage that they can be synthesized in aqueous solutions in large quantities and high yields across a wide range of dimensions, and copper is an earth-abundant template. The Wiley lab developed a variety of methods to coat copper nanowires in solution with shells of Ni, Co, Zn, Sn, In, Ag, Au, and Pt (see Figure 1 and Acc. Chem. Res., 2016). We showed that a 20-nm-thick shell of nickel can increase the time for the sheet resistance of Cu nanowire networks to double from 3 days to 400 years. The resistance of core-shell Cu-Ni nanowires to corrosion allowed them to replace the costly indium tin oxide electrode in an organic solar cell while achieving a nearly equivalent efficiency. We subsequently coated copper nanowires with oxidation-resistant shells of Sn, Zn, and In through electrochemical methods, and oxidized these shells to make them transparent and thereby improve the optoelectronic properties of the nanowire film. This work established the chemical methods necessary for creating a variety of metallic nanowire materials from copper nanowire templates.
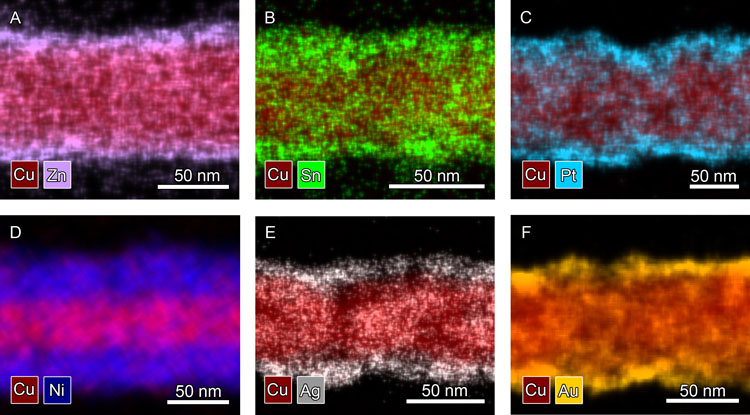
Figure 1. X-ray spectroscopy images of (A) Cu-Zn, (B) Cu-Sn, (C) Cu-Pt, (D)Cu-Ni, (E) Cu-Ag, and (F) Cu-Au core-shell nanowires. See the paper for more details
Shells of Ni, Zn, Sn, and In protect the copper nanowires from corrosion, but the creation of conductive copper nanowire films still requires annealing with hydrogen or treatment with acid to remove the copper oxide shell. We hypothesized this problem of high contact resistance could be solved by coating copper nanowires with metals that do not form surface oxides. We developed a novel way to coat copper nanowires with shells of Ag, Au, and Pt while preventing galvanic etching of the nanowires. Silver-coated copper nanowires were conductive immediately after printing without the need for additional post-processing, and had optoelectronic properties equivalent to films of silver nanowires. The ability of silver-coated copper nanowires to make conductive networks without post-processing has enabled their use in conductive composites. This work solved the problems of (1) how to coat copper nanostructures with a more noble metal while preventing galvanic etching and (2) how to achieve a low contact resistance between copper nanowires without any post-processing.
Related Publications:
Chen, Z.; Rathmell, A. R.; Ye, S.; Wilson, A. R.; Wiley, B. J. Optically Transparent Water Oxidation Catalysts Based on Copper Nanowires. Angew. Chem. Int. Ed. 2013, 52, 13708-13711.
Stewart, I.E.; Rathmell, A.R.; Yan, L.; Ye, S.; Flowers, P.F.; You, W. Wiley, B.J. Solution-Processed Copper-Nickel Nanowire Anodes for Organic Solar Cells. Nanoscale, 2014, 6, 5980-5988.
Chen, Z.; Ye, S.; Stewart, I.E.; Wiley, B.J. Copper Nanowire Networks with Transparent Oxide Shells That Prevent Oxidation without Reducing Transmittance. ACS Nano, 2014, 8, 9673-9679.
Stewart, I.E.; Ye, S.; Chen, Z.; Flowers, P.F.; Wiley, B.J. Synthesis of Cu-Ag, Cu-Au, and Cu-Pt Core-Shell Nanowires and Their Use in Transparent Conducting Films. Chem. Mater., 2015, 27, 7788-7794.
Flowers, P.F.; Catenacci, M.J.; Wiley, B.J.; High-speed, Solution-Coatable Memory Based on Cu–SiO2 Core–Shell nanowires. Nanoscale Horiz. 2016, 1, 313-316.